Antibiotic Guidelines in a Nutshell
Staphylococcus aureus
Cloxacillin remains the drug of choice for all S. aureus infections where the isolate is deemed to be clinically significant. The percentage of methicillin-resistant Staphylococcus aureus (MRSA) strains detected by Lancet Laboratories in Johannesburg during 2014 was less than 20% from all specimens and 27% from blood cultures. Cloxacillin resistance is now detected in both hospital- and community-acquired isolates of S. aureus. Strains of S. aureus that are cloxacillin resistant are insensitive to all currently available penicillins and cephalosporins. The highest burden of MRSAs is found in long-term care facilities, patients who require chronic care, trauma units and large ICUs. These MRSAs are usually multi-drug resistant and treatment is often limited to the glycopeptides and linezolid. All of our reported S. aureus cultures are vancomycin, teicoplanin and linezolid susceptible. However, there are reports of resistance to all three of these antibiotics in other parts of the world. Fortunately this is not yet a problem in South Africa.
Coagulase Negative Staphylococci (CNS)
Most CNS isolates are skin contaminants. In recent years, however, these organisms have emerged as signicant causes of healthcare-associated infections. Staphylococcus epidermidis isolates are very often methicillin-resistant. Therefore, in patients with serious infections (e.g. infected prostheses or endocarditis) or with line sepsis, the empiric therapy for a clinically signicant CNS infection would be vancomycin, teicoplanin or linezolid. Resistance to these antibiotics are gradually increasing worldwide and treatment should be based on the antibiogram results. Please de-escalate as soon as possible when the antibiotic sensitivity of the isolate is known.
Streptococcus pneumoniae
Penicillin resistance in S. pneumoniae isolates occurs due to changes in the penicillin-binding proteins (PBPs) of the bacterial cell wall, resulting in decreased afnity for penicillins and consequently a stepwise resistance to members of this antibiotic class. At a low minimum inhibitory quotient (MIC), this resistance can be overcome by increasing the dose of the beta-lactam antibiotic. Closed infections such as meningitis require higher doses of antibiotic to achieve adequate levels in the infected area. Thus higher MIC breakpoints are used to determine susceptibility for CSF specimens than for lower respiratory tract specimens and bloodstream infections.
The recommended empiric treatment for suspected pneumococcal meningitis remains a 3rd generation cephalosporin. In the event of intermediate cephalosporin resistance from a CSF isolate, the maximum dose of ceftriaxone (adults 4 g daily, children 50 ? 75 mg/kg daily) or cefotaxime (adults 4 g daily, children 50 mg/kg/dose 6 hourly) should be used in combination with another agent e.g. vancomycin or rifampicin. In the event of a fully resistant isolate from the CSF (luckily none have been reported locally) vancomycin is the drug of choice.
In isolates from the upper and lower respiratory tract displaying intermediate resistance to penicillin and/or 3rd generation cephalosporins, resistance can be overcome by dosing maximally with the chosen beta-lactam agent. Erythromycin sensitivity in lower respiratory tract specimens has been below 50% during the past few years. Levooxacin and moxioxacin and telithromycin are all still 100% active against beta-lactam resistant strains.
Enterococcus faecalis / Enterococcus faecium
Prior to treatment of enterococcal infections, all intravenous lines, intra-arterial catheters, and urinary catheters should be removed if possible. Infections for which a single antibiotic directed against enterococci can be used include urinary tract infections (UTIs), most intra-abdominal infections, and uncomplicated wound infections. Combination therapy with a cell wallactive agent (e.g. betalactam) and a synergistic aminoglycoside should be considered for treating serious enterococcal infections in critically ill patients, and in those with evidence of sepsis, as well as in patients with endocarditis, meningitis, osteomyelitis, or joint infections.
Ampicillin is the drug of choice for monotherapy of susceptible E. faecalis infection. For rare strains that are resistant to ampicillin because of beta-lactamase production, amoxicillin-clavulanate may be used. Vancomycin should be used in patients with a penicillin allergy or infections with strains that have high-level penicillin resistance due to altered PBPs.
Nitrofurantoin is effective in the treatment of enterococcal UTIs, including many caused by vancomycin-resistant enterococci (VRE). Linezolid, daptomycin, and tigecycline, are the agents of choice for treating VRE infections
Extended-spectrum beta-lactamase (ESBL) Production
ESBLs are genes carried on plasmids and thus easily transferable from one member of the Enterobacteriaciae family to another. ESBLs were rst isolated in Klebsiella species, but have rapidly spread to all the Enterobacteriaciae. These bacteria are resistant to ampicillin, all cephalosporins (including the 4th generation cephalosporins) and pipericillin/tazobactam.
Infection control measures should be instituted for all patients with ESBL positive organisms.
The only antibiotic class currently proven to be effective in the treatment of severe infections caused by ESBL-producing organisms is the carbapenems (imipenem, meropenem, doripenem, and ertapenem). There are no clear differences in efcacy between imipenem and meropenem. The choice of one over the other is predominantly based on toxicity proles in specic hosts. Clinical data suggest that the efcacy of doripenem is equivalent to that of imipenem and meropenem. Ertapenem has the advantage of once-daily dosing and has good in vitro activity, but is reserved for treating susceptible ESBL-producing organisms that are not associated with severe sepsis.
ESBL-producing isolates typically show greater than average resistance to other agents, including aminoglycosides and uoroquinolones. Tigecycline, a tetracycline derivative, is a potential alternative for the treatment of ESBL-producing organisms, especially for patients with beta-lactam allergies.
Carbapenemase Producing Enterobacteriaciae (CPE) and Carbapenem-Resistant Enterobacteriaciae (CRE)
For years, carbapenems have been used successfully to treat infections due to resistant Enterobacteriaceae, such as Escherichia coli and Klebsiella pneumoniae, including those producing ESBLs. However, carbapenem-resistant Enterobacteriaceae have recently emerged. This is most commonly due to the production of a carbapenemase enzyme which confers broad resistance to most beta-lactam antibiotics, including the so-called “last-line” carbapenems. Carbapenem resistance can also be conferred when porin deciencies (which restrict the entry of the beta-lactam antibiotics into the cell) are combined with ESBLs. The prevalence of CRE infections has increased over the last decade, especially in healthcare settings. CREs are now recognized by the US Centers for Disease Control and Prevention as well as other international healthcare agencies as an urgent public health threat.
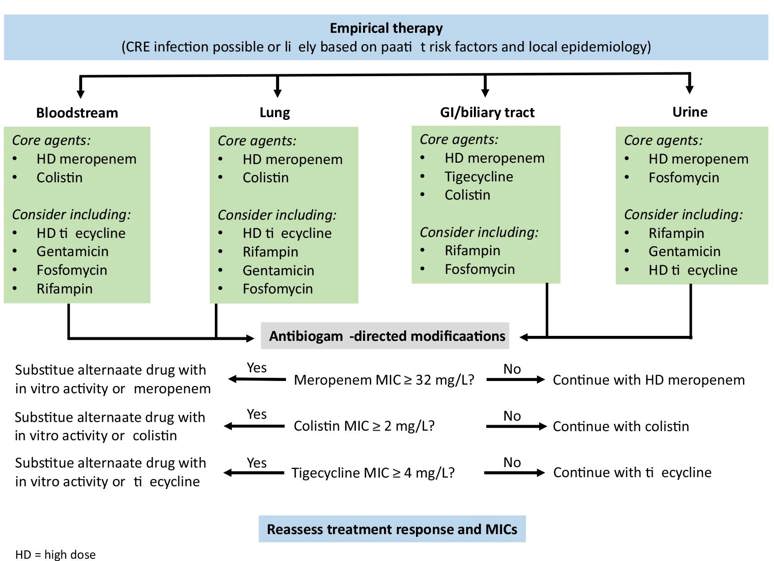
The optimal treatment of CRE infections is uncertain. Since carbapenem-resistant organisms are often resistant to other antimicrobial classes, including most beta-lactams, additional susceptibility tests are undertaken for antimicrobials such as colistin, tigecycline, aztreonam and rifampicin. The following antimicrobial therapies have been used with various levels of success in the treatment of CRE infections:
- Colistin (polymyxin E) - colistin monotherapy is NOT recommended.
- Carbapenems have been used, though counter-intuitively, in the treatment of infections with CRE, usually as the adjuvant component of a combination drug regimen. This strategy is potentially useful ONLY when the MICs of the infecting carbapenemresistant organisms are still relatively low (i.e. not more than 4 to 16 mg/L)
- Tigecycline has been used in the treatment of infections with CRE, primarily as an adjuvant drug in combination with other antibiotics.
Recently the following treatment algorithm using combination therapy for the treatment of CRE infections was proposed, based on the site of infection and antibiogram results².
Empiric Treatment of Community-acquired Urinary Tract Infections
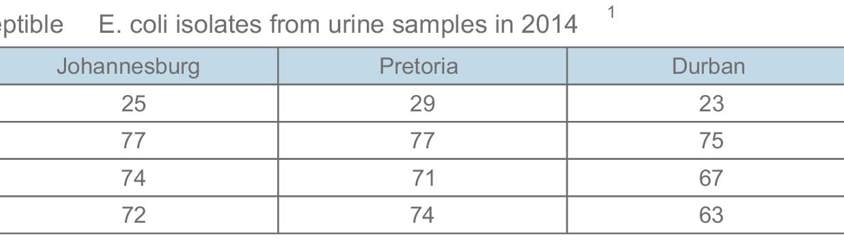
The empiric antibiotic of choice in the treatment of a community-acquired UTI remains amoxicillin-clavulanate or a 2nd generation cephalosporin. Please note that in 2014, 28% of E. coli isolates from the urinary tract were resistant to ciprooxacin in Johannesburg (26% in Pretoria and Cape Town, and 37% in Durban). This high rate of quinolone resistance should be kept in mind when empirically prescribing these agents to treat a community-acquired UTI.
Another option for the treatment of uncomplicated lower urinary tract infections (but ONLY where the organism retains susceptibility) is fosfomycin, which is a urinary antiseptic. Nitrofurantoin (also a urinary antiseptic) may also be considered, but is frequently out of stock in South Africa.
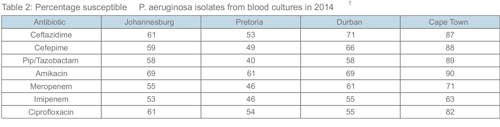
Pseudomonas aeruginosa
There are some strains of P. aeruginosa in the Gauteng region which are fully resistant. Colistin sensitivity is routinely tested, but there are practical problems with this antibiotic, and some strains are inherently resistant. In view of the rapidly increasing ESBL problem, the carbapenems have become the empiric choice in many ICU patients with suspected nosocomial Gramnegative infections. Please note that some Pseudomonas strains have isolated imipenem / meropenem resistance. In addition ertapenem has no activity against Pseudomonas aeruginosa, Acinetobacter baumanii, Burkholderia cepacia and Stenotrophomonas maltophilia.
AmpC producing bacteria
Certain organisms (e.g. Enterobacter, Serratia, Citrobacter, Morganella and Providencia species) have a chromosomal gene that encodes inducible resistance to the 1st, 2nd and 3rd generation cephalosporins as well as ampicillin. Therefore the abovementioned antibiotics cannot be used for treatment of infections with these bacteria. Empiric therapy for all AmpC producing bacteria includes quinolones, the 4th generation cephalosporins (e.g. cefepime) or the carbapenems (e.g. meropenem, imipenem and ertapenem).
References:
1. South African Society for Clinical Microbiology. SASCM Laboratory Surveillance Private Sector Data January to December 2014. Available at: http://www.dssa.co.za/images/SASCM_2014_Consolidated_Data.pdf
2. Petrosillo N, et al. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther 2013; 11(2): 159 - 177.
3. Morrill HJ, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2015; 2(2): 1 - 15.
