Cervical Cancer Screening
Cervical cancer is caused by chronic infection with one of 14 high-risk human papillomaviruses (HR-HPV). Most infected women will clear HR-HPV through natural immunity (usually between 1 - 2 years of acquisition), but a proportion (± 10%) will have infection that persists, resulting in cellular changes that may progress from premalignant dysplasia to high grade dysplasia (CIN2, CIN3), and eventually to invasive cervical carcinoma. The time line from exposure to the development of invasive carcinoma in the persistently infected, undetected woman is 10 - 15 years, though this may be shortened by concurrent HIV infection.
Why should I offer Cervical Cancer screening to my female patients?
Sexually transmitted infection with HPV is very common, with most women being exposed to HPV at some point. It is estimated that every year 4,802 Kenyan women are diagnosed with cervical cancer and 2,451 die from this preventable disease. It is the second most frequent cancer amongst women and the most frequent in women aged 15 - 44 years. HPV-16 and -18 are responsible for 63.9% of invasive cervical cancer cases. Approximately 3.2% of women are estimated to be infected with HPV-16 and -18 at any given time.
With appropriate screening, cervical cancer can be prevented, by detecting pre-cancerous lesions, or cured, by detecting cancer early and managing appropriately. Cervical cancer is one of the only cancers that can be prevented through appropriate screening with readily available tests and minimally invasive procedures.
What is the most appropriate strategy for cervical cancer screening?
The Ministry of Health's official position is captured in the National Cancer Screening Guidelines published by the Ministry of Health in November 2018 and launched by the CS for Health in February this year. The full document can be found on the link below.
https://www.health.go.ke/wp-content/uploads/2019/02/National-Cancer-Screening-Guidelines-2018.pdf
Who should be screened and when?
- Any woman who has ever had sexual intercourse is eligible for cervical cancer screening
- The target population for screening with HPV testing is women aged 25 to 49 years
- Women aged 50-65 years are still at risk of cervical cancer and can therefore receive screening but with longer intervals between each test.
- Screening interval is 5 years for any women who tests negative for HPV
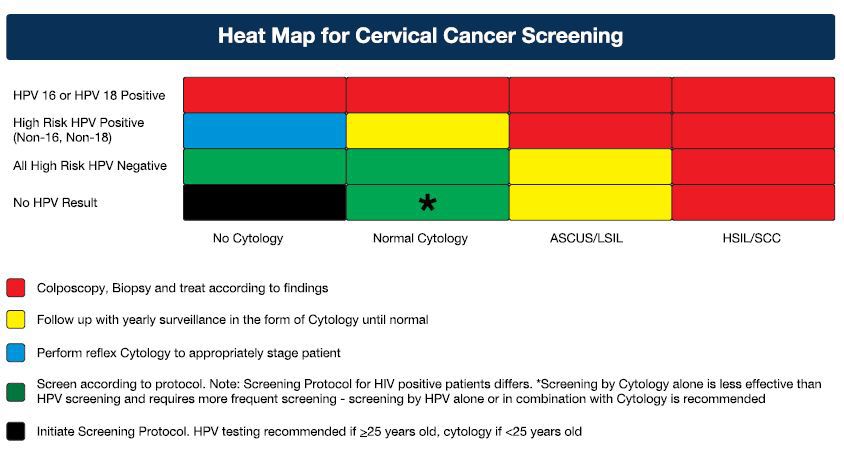
Screening methods for cervical cancer
For the Kenyan screening program the National Cancer Screening Guideline recommends the following:
- HPV testing is recommended as the primary screening method for women above 30 years of age.
- Where HPV testing is not yet available, or loss-to-follow-up is a risk, then Visual Inspection with Acetic acid (VIA) or Visual Inspection with Acetic acid and Visual Inspection with Lugol's iodine (VIA/VILI) is recommended as the primary screening method.
- Pap smear is recommended as a primary screening method in the following situations:
- For women not eligible for VIA or VIA/VILI because their squamo-columnar junction (SCJ) is not visible, and HPV screening not accessible
- As a primary test in women under 30 years of age
- As a co-test with HPV in HIV positive women where the resources are available
It is worthy to note that the guidelines are based on a sexual debut from 18-21 years. Women who had an earlier sexual debut should be screened with HPV earlier as HPV testing is recommended 5-years post-sexual debut.
Therefore HPV can be offered to women from 25 years of age who had sexual debut before the age of 20 years.
Lancet Laboratories offers the most affordable HPV testing available. Please contact your Lancet Marketer for more information.
Self collection sampling (Evalyn-brush)
Pathologist Lancet Kenya has introduced a self-collection kit that any woman can use to collect a sample by herself for HPV testing.
- The self-collection kit is Evalyn Brush ® that is specially patented for collecting material from the vagina
- The Evalyn-brush has soft fine bristles, ensuring that enough fluid and cell material is collected for testing in the lab. The Evalynbrush ® was designed by women for women
- Self-collected sample using the Evalyn-brush has been confirmed to be as accurate as cervical sample collected by a gynaecologist or a nurse
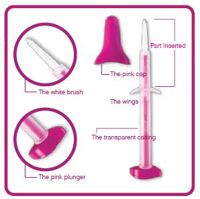
Components of the Evalyn-Brush
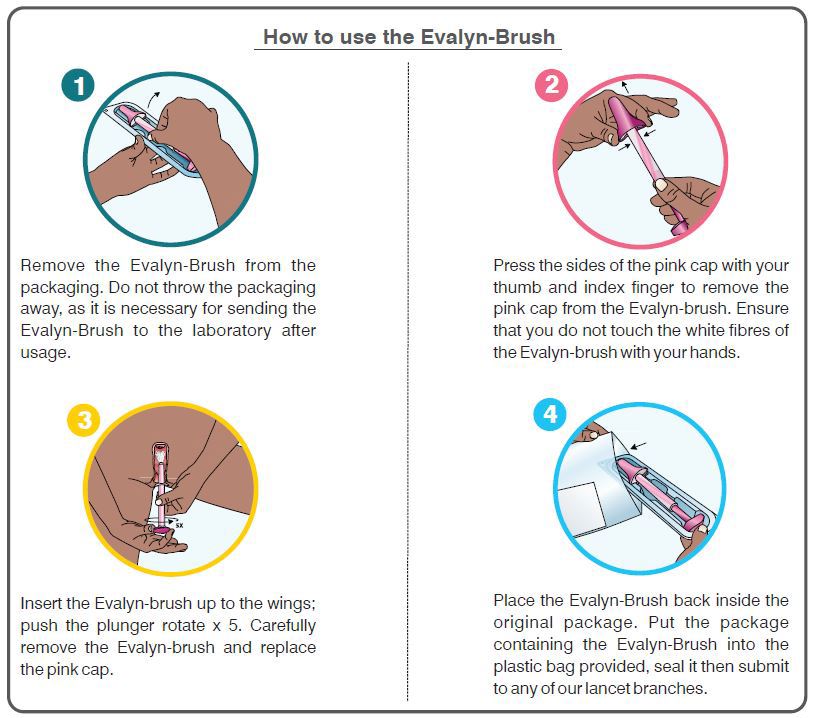
- The Evalyn-brush is approx.20cm long and consists of a transparent casing with wings. Within the casing is a pink stick consisting of a plunger at one end and white bristles at the other end covered by a pink cap. The pink cap is removed before insertion.
- The bristles are exposed by pushing the plunger when inserted into the vagina. The Evalyn-brush is inserted as far into the vagina up to the wings.
References
- Andrae B, Kemetli L, Sparén P et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst 2008, 100; 9:622-629
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older; a subanalysis of the ATHENA study [published online August 23, 2011] Lancet Oncol. doi;10.1016/S1470-2045(11)70188-7
- Cobas® HPV test [package insert]. Indianapolis, IN: Roche Diagnostic; 20145. Presented to FDA 12 March 2014: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/ucm388531.htm
- http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/medicaldevices/me dicaldeviceadvisorycommitte/microbiologydevicespanel/ucm388565.pdf
- http://www.hpvcentre.net/statistics/reports/ZAF FS.pdf
- http:www.hpv16andhpv18.com
- http://seer.cancer.gov/archive/csr/1975 2010/#contents
- Leyde WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst 2005, 97; 9:675-683
- Wright TC Jr, Stoler CM, Apple R, Derrion T, Wright TL. The ATHENA human papillomavirus study; design, methods and baseline results. AmJ Obstet Gynecol 2012, 206; 1:46.e1-46.e11
